Abstract
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is a common hematological malignancy with a high unfavorable prognosis. Ubiquitin-specific-processing protease 7 (USP7) is one of the deubiquitinating enzymes attracting concentrated attention in current studies for cancers; it is also involved in regulation of oncogenic transcriptional program in T-ALL, and serves as a potential target to treat T-ALL. USP7 is interacted with PHD Finger protein 8 (PHF8), a histone lysine demethylase in T-ALL. However, the impact of the interaction in T-ALL development is undetermined. In this study, we explored the anti-tumor effect of targeting USP7 and its downstream substrate in T-ALL and underlying mechanism.
Methods
In vitro GST pull-down assay, co-immunoprecipitation assay, protein deubiquitination and stability assays were used to detect the interaction of USP7 with its direct substrate and the ubiquitination level. Gain-of-function(overexpression) and loss-of-function (lentiviral shRNA knockdown) approaches for USP7 were conducted for gene expression, CCK-8 cell proliferation assay, and apoptosis assay with annexin V + PI staining. ChIP-qPCR assay was used to explore the enrichment of histone markers in promoter region of target gene. In vivo human T-ALL xenograft mouse model was developed in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mouse by tail vein injection of 2.5 × 10 6 /mouse and the leukemia engraftment was evaluated with human CD45+ cells by flow cytometry. The qPCR and western blot were carried out for detection mRNA level and protein level of related genes. The mRNA level of USP7 and its substrate PHF8 was examined by qPCR in 43 newly-diagnosed T-ALL patients with an approval of the Ethics Committee. Microarray datasets of T-ALL patients from GEO database were used to determine the USP7 and its substrate -related genes with web-based platform MEM and Limma.
Results
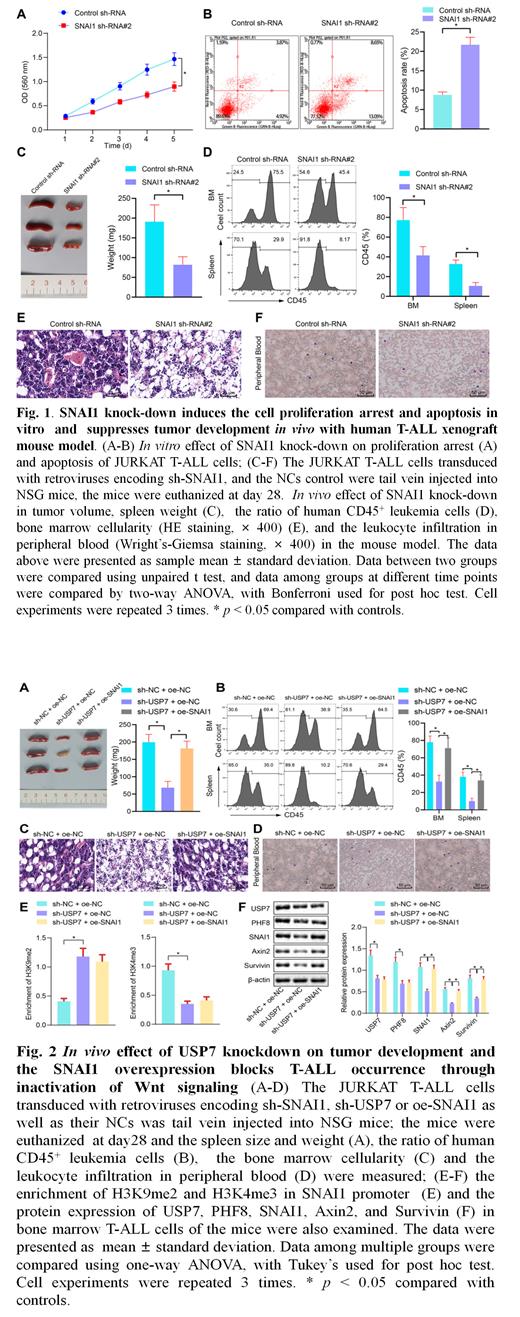
Results showed that USP7 directly interacted with PHF8; and overexpressed USP7 deubiquitinated and stabilized PHF8, and enhanced cell proliferation of JURKAT T-ALL cells. These data indicates targeting USP7 promotes cell proliferation of T-ALL cells through its substrate PHF8. Moreover, SNAI1 was identified as one of key co-expressed genes with PHF8 in microarray datasets from T-ALL patients, and knock-down of PHF8 suppressed the SNAL1 expression and increased the recruitment of repressive histone marker, H3K9me2 but decreased the enrichment of H3K4me3 in SNAI1 promoter. SNAI1 knockdown significantly induced the cell proliferation arrest and apoptosis of T-ALL cells (Fig.1A & 1B). With in vivo human leukemia xenograft mouse model, we further demonstrated that SNAI1 knockdown significantly reduce the spleen size and weight, the ratio of human CD45(+) cells, bone marrow cellularity and also the inflammatory cell infiltration compared to the scramble shRNA control (Fig.1C-1F). Moreover, Wnt signaling was identified as SNAI1 interaction partners by the pathway analysis in the SNAI1 interaction genes in the microarray data from T-ALL patients. SNAI1 knockdown significantly suppressed the expression of the Axin2 and Survivn, the key components of Wnt signaling. These data suggested oncogenic role of USP7/PHF8/SNAI1/Wnt signaling in T-ALL. Next, we explored the effect of SNAI1 on USP7 knockdown-induced anti-tumor effect. We found that USP7 knockdown induced cell proliferation arrest and apoptosis, and overexpression of SNAI1 could block the effect in vitro. Furthermore, the in vivo data showed that USP7 knockdown significantly suppressed spleen size and weight, the ratio of human CD45(+) cells, bone marrow cellularity, inflammatory cell infiltration, and the protein expression of PHF8, SNAI1, Axin2 and Survivin; and SNAI1 overexpression completely rescue the USP7 knockdown-mediated antitumor effect and restored the expression of Axin2 and Survivin in vivo (Fig.2).
Conclusion:
Our results demonstrated that targeting USP7 by shRNA induces the cell proliferation arrest and apoptosis by promoting ubiquitination of PHF8 and suppressing SNAI1/Wnt signaling in T-ALL. Our data also revealed the oncogenic roles of USP7/PHF8/SNAI1/Wnt signaling in T-ALL and suggested targeting the signaling pathway as potential therapy in T-ALL.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal